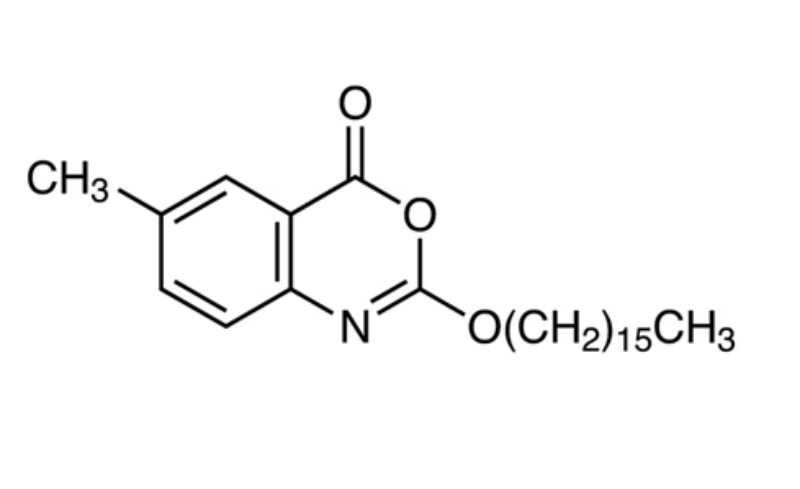
Cetilistat Cas number: 282526-98-1 Molecular Formula:C25H39NO3
| Melting Point | 72.0 to 76.0 °C |
| Density | 1.02 |
| storage temp | Sealed in dry,2-8°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| optical activity | N/A |
| Appearance | Off-white Cryst |
| Purity | ≥98% |
Cetilistat (also known as ATL-962) was approved in September 2013 by the Japanese Ministry of Health, Labor and Welfare for the treatment of obesity, limited to patients with both type 2 diabetes mellitus (T2DM) and dyslipidemia, and with a body mass index (BMI)25 kg/m2 in spite of dietary treatment and/or exercise therapy. As with orlistat, cetilistat works via inhibition of pancreatic lipases in the gut to inhibit fat absorption and thereby reduce caloric uptake from diet. The medicinal chemistry program has not been described in the scientific literature, but the patent describing cetilistat also describes the synthesis of analogs with varied aryl substituents and lipophilic tails. The synthesis of cetilistat involves condensation of a hexadecylcarbonochloridate with 2-amino-5-methylbenzoic acid; other analogs were synthesized by varying the carbonochloridate and 2-aminobenzoic acid components. Cetilistat is a potent inhibitor of human and rat pancreatic lipase with IC50s of 15 and 136 nM, respectively, with little inhibition of trypsin or chymotrypsin.
A novel pancreatic lipase inhibitor for the treatment of obesity in both diabetic and non-diabetic patients.
Ask Doctor advise first